
The painting above shows a crescentic glomerulonephritis and necrotizing arteritis. A mixed interstitial inflammatory infiltrate is shown in the top left corner, and tubules are dilated with a few red blood cell casts. This cluster of findings can occur in ANCA-associated disease. Renal biopsy images representing manifestations of ANCA-associated glomerulonephritis are shown below. The main differential diagnosis for crescents within glomeruli includes immune complex glomerulonephritides, anti-GBM nephritis, and ANCA-associated glomerulonephritis (pauci-immune), although nearly any glomerulonephritis with mesangial and/or endocapillary proliferation can produce crescents (examples – infection-associated glomerulonephritis, fibrillary glomerulonephritis, and others). Correlation with ANCA serologies is recommended in any case of a crescentic glomerulonephritis.
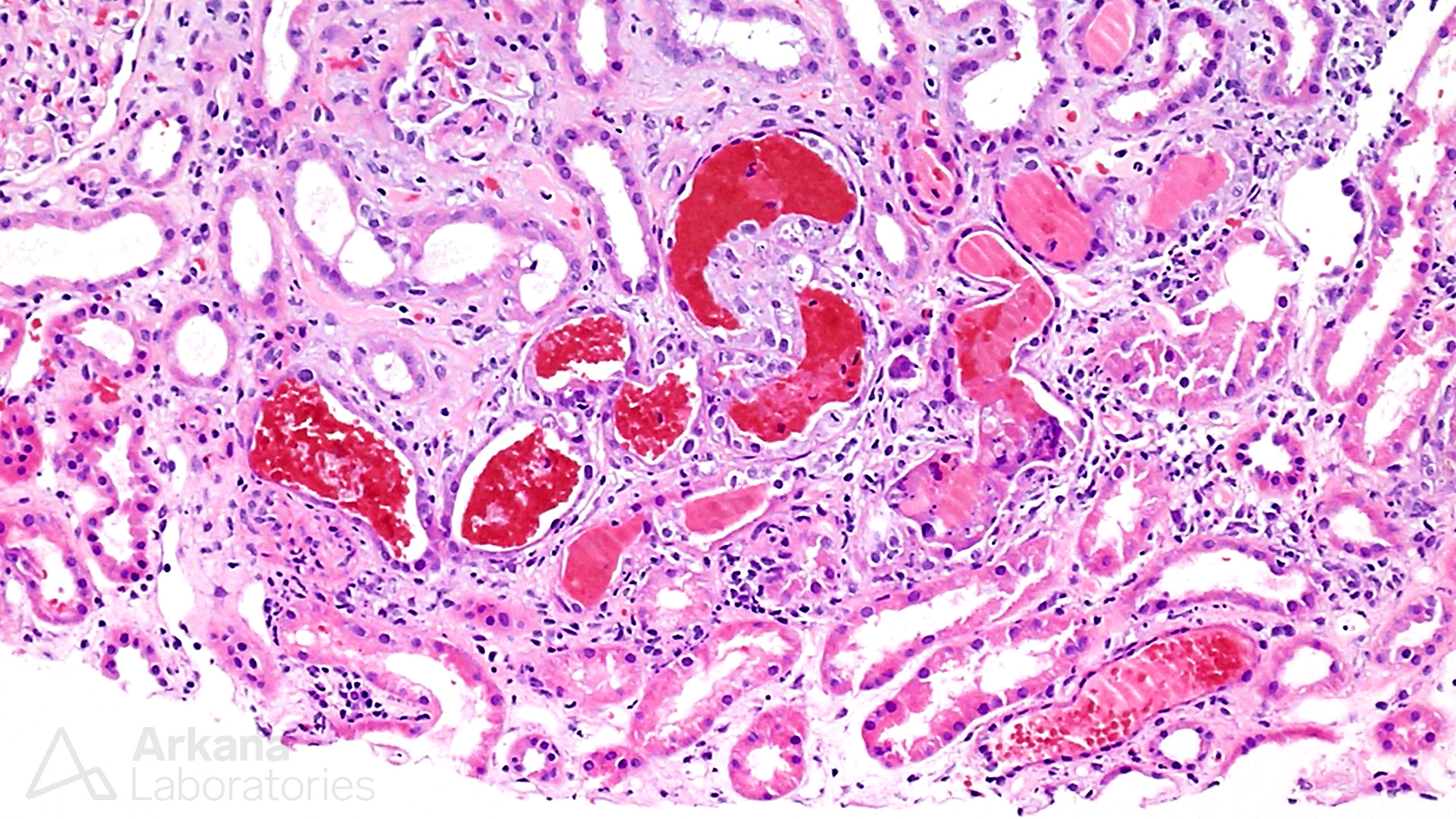
Fresh and hemolyzing red blood cell casts in ANCA-associated glomerulonephritis.
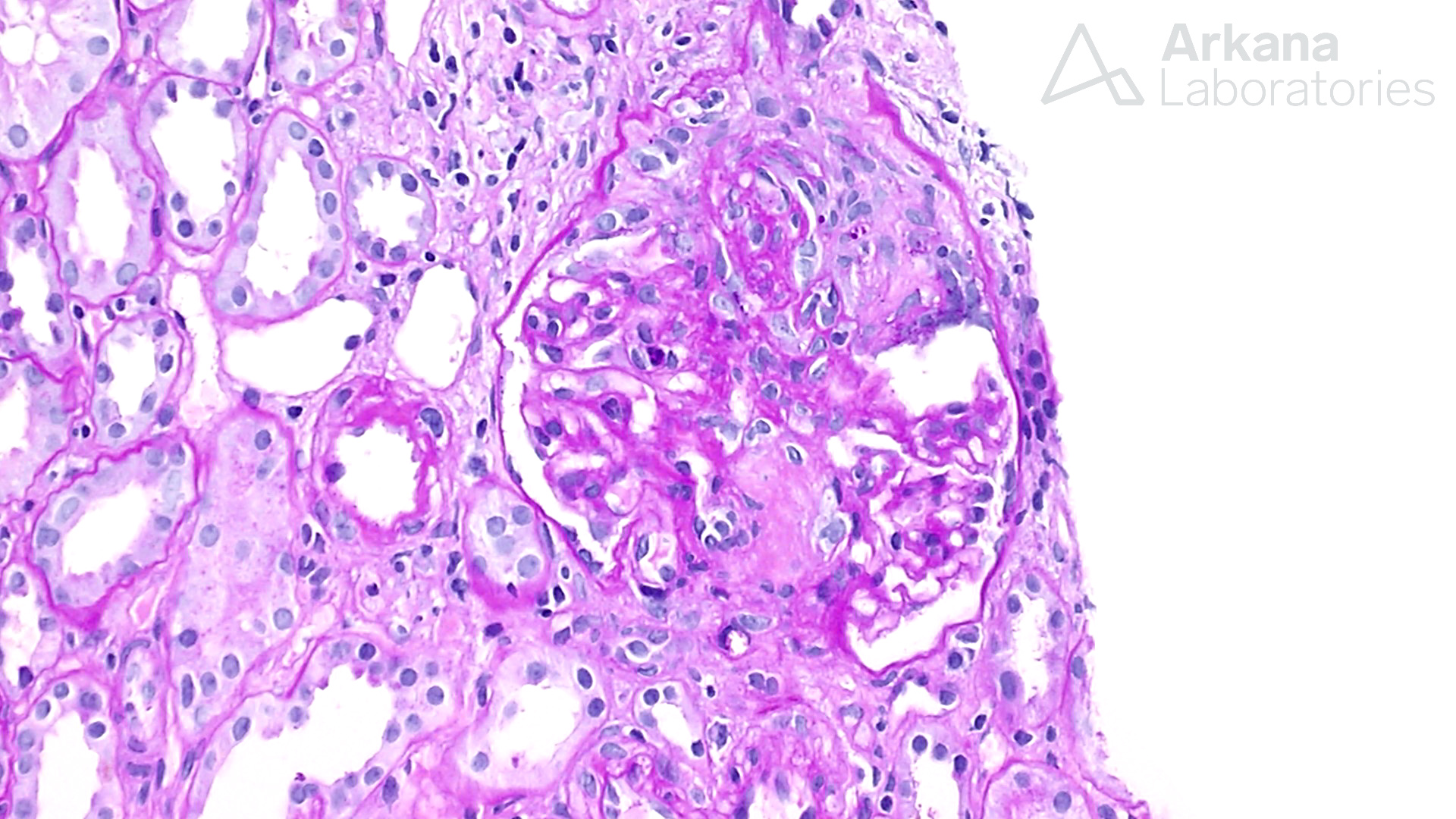
Cellular crescent in a case of ANCA-associated glomerulonephritis.
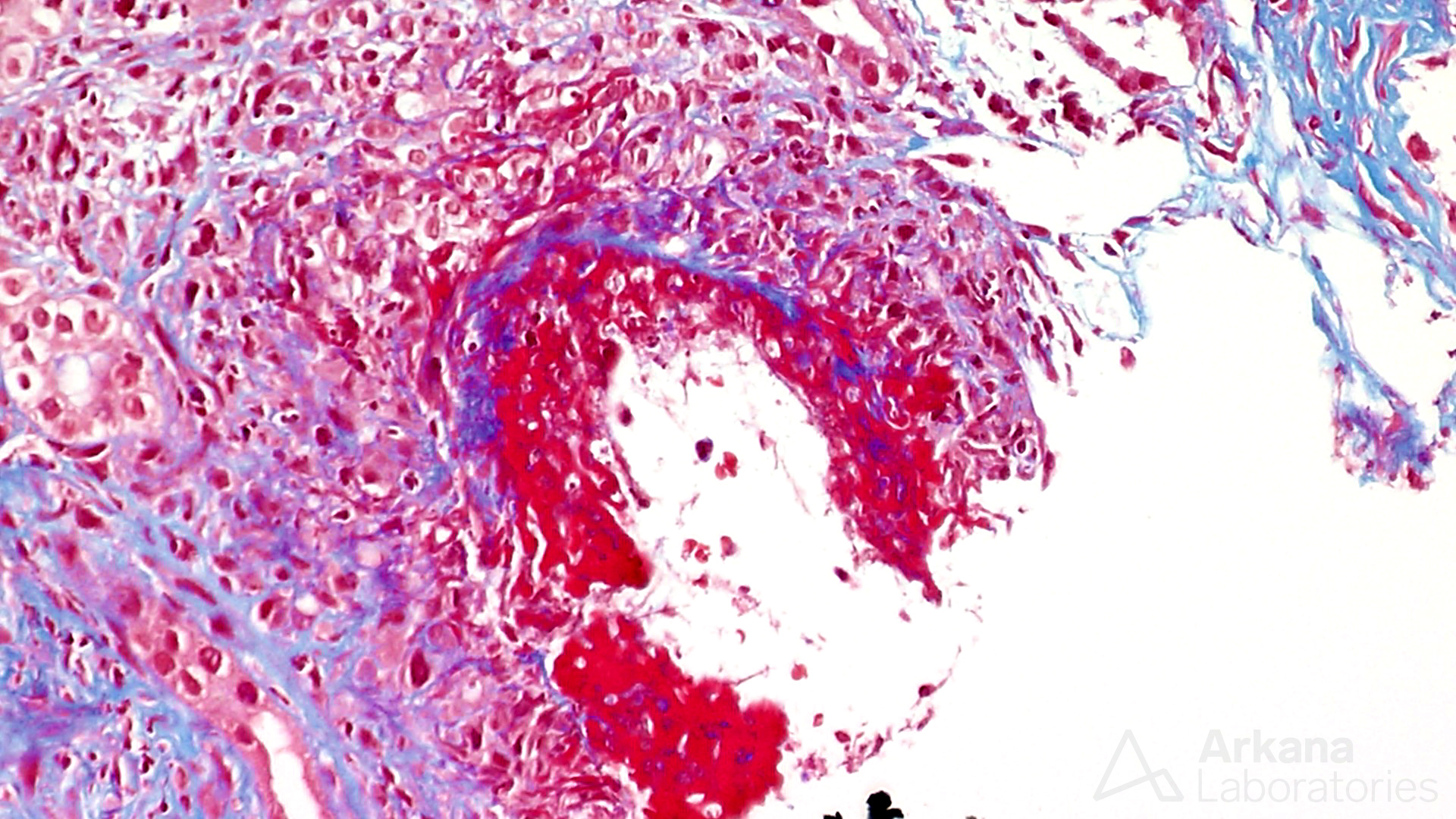
Transmural necrotizing arteritis in a case of ANCA-associated glomerulonephritis.
The spectrum of ANCA-associated renal disease includes granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and microscopic polyangiitis (Jennette JC et al, 2017). Additionally, infections can trigger production of ANCAs and cause an ANCA-associated glomerulonephritis. Membranous glomerulopathy can also occur in the setting of an ANCA-associated necrotizing and crescentic glomerulonephritis, due to anti-MPO immune complexes deposited at the subepithelial space of glomeruli (Nasr et al, 2009).
In a recent prospective cohort study that included 115 patients with this disease, it was found that the percentage of normal intact glomeruli that lacked crescents, necrosis, or scarring of the capillary tuft, was the most important factor that could predict the risk of progression of end-stage kidney disease at the time of diagnosis. When combined with the patient’s glomerular filtration rate and the amount of interstitial fibrosis and tubular atrophy (IF/TA) in the biopsy, an ANCA renal risk score was developed (Brix SR et al, 2018). Assessing a ANCA renal risk score requires the amount of normal glomeruli (with cutoffs of >25%, 10-25%, and <10%), the amount of IF/TA (<25% or >25%), and the GFR at the time of biopsy (> 15 mL/min or <15 mL/min). An app that can be accessed on smartphones was created for nephrologists to be used for risk prediction by this group and is called “ANCA Score” (Brix SR et al). This renal risk score was validated prospectively in a group of 205 patients and was shown to stratify patients, showing differing percentages of end-stage kidney disease at 36 months (low risk 0%, moderate risk 27%, high risk 78%). It is suggested that this formula can help nephrologists decide which patients should receive cytotoxic therapies, versus those that may be spared as their risk of end-stage renal disease, is too high that the risks of treatment may outweigh the benefit (Kronbichler A et al, 2018).
References:
Brix SR, Noriega M, Tennstedt P, Vettorazzi E, Busch M, Nitschke M, Jabs WJ, Ozcan F, Wendt R, Hausberg M, Sellin L, Panzer U, Huber TB, Waldherr R, Hopfer H, Stahl RAK, Wiech T. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney International 2018 Dec; 94 (6): 1177-1188.
https://www.ncbi.nlm.nih.gov/pubmed/30385041
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.

