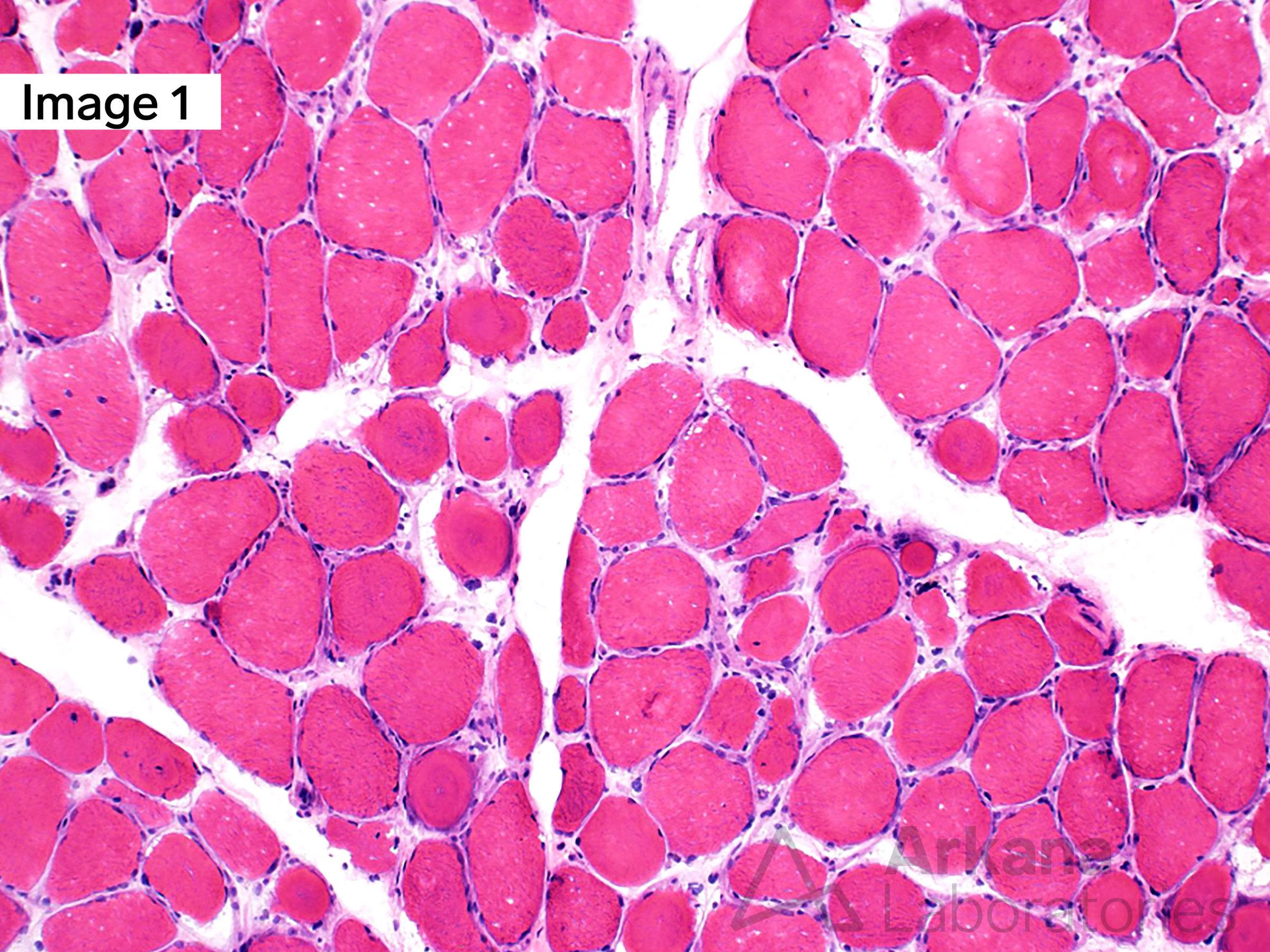
A 55-year-old male with Myotonic Dystrophy Type 2 (DM2). Frozen sections of skeletal muscle stained with H&E show moderate to marked variation in muscle fiber size with scattered ringbinden (ring fibers), a few nuclear bag muscle fibers, and minimally increased muscle fibers with internalized nuclei (1). Ultrastructural examination shows a ring fiber with disorientation of peripheral myofibrils running perpendicularly to the central myofibrils of the muscle fiber (2). (Original magnifications: A. Hematoxylin and eosin (H&E)-stained frozen section of skeletal muscle, 100x; B. Electron microscopy, 1000x)
Using the images, match the diagnosis and genetic alteration in this common muscular dystrophy of adulthood.
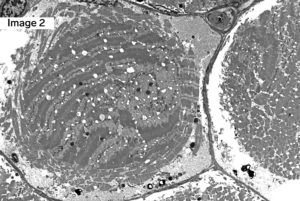
Answer: DM2, ZNF9
Myotonic dystrophy type 2 (DM2), also known as proximal myotonic myopathy (PROMM) which is caused by expanded CCTG repeats in intron 1 of the zinger finger protein 9 gene (ZNF9) on chromosome 3q.
The expanded repeats in the noncoding regions are transcribed into RNAs which produce a toxic gain-of-function to deregulate several RNA binding proteins, resulting in aberrant RNA slicing, polyadenylation, or expression of many genes.
There is no cure for myotonic dystrophy and definitive diagnosis is made by genetic testing, although muscle biopsy may be helpful to guide the choice of targeted gene panel testing.
Myotonic dystrophy is an autosomal dominant disease, with myotonic dystrophy type 1 (DM1) being the most common adult muscular dystrophy, due to expanded CTG repeated in the untranslated region of the myotonic dystrophy protein kinase gene (DMPK) on chromosome 19q13.
Why were the other answers wrong?
Becker Muscular Dystrophy (BMD) is caused by alterations in the DMD gene; however, this spectrum of disease is not seen in the image, the clinical presentation does not match, and it is not as common as DM2.
Central Core Myopathy/Disease (CCD) is caused by alterations in the RYR1 gene; however, this spectrum of disease is not seen in the image, the clinical presentation does not match, and it is not as common as DM2.
McArdle Disease (Glycogen Storage Disease, Type V) is caused by alterations in the PYGM gene and commonly presents with exercise intolerance. Among metabolic myopathies of carbohydrate metabolism, it is the most common.
References
Brook JD, McCurrach ME, Harley HG, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799-808. PMID: 1310900.
Lawal TA, Todd JJ, Witherspoon JW, et al. Ryanodine receptor 1-related disorders: an historical perspective and proposal for a unified nomenclature. Skelet Muscle. 2020 Nov 16;10(1):32. PMID: 33190635.
Liquori CL, Ricker K, Moseley ML, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001 Aug 3;293(5531):864-7. PMID: 11486088.
Nogales-Gadea G, Brull A, Santalla A, et al. McArdle Disease: Update of Reported Mutations and Polymorphisms in the PYGM Gene. Hum Mutat. 2015 Jul;36(7):669-78. PMID: 25914343.
Thornton CA. Myotonic dystrophy. Neurol Clin. 2014 Aug;32(3):705-19, viii. PMID: 25037086.
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.