
The painting above depicts membranous glomerulopathy. A single glomerular capillary loop with confluent subepithelial and intramembranous electron dense deposits along the glomerular basement membrane is shown. Podocytes are showing foot process effacement and microvillous transformation, which results in loss of the filtration barrier leading to nephrotic syndrome. An electron micrograph from a patient with membranous glomerulopathy is shown below.
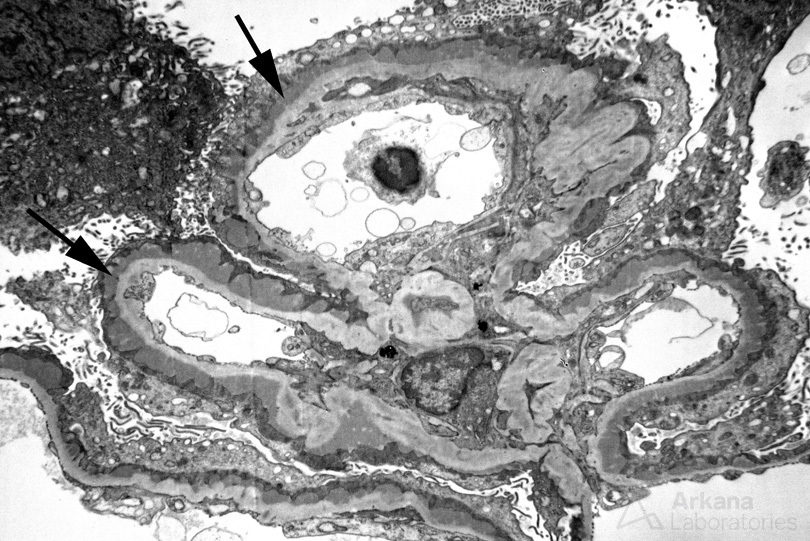
Membranous glomerulopathy is the second most common cause of nephrotic syndrome in adults. A majority of cases are considered primary with autoantibodies directed against the podocyte antigens phospholipase A2 receptor (PLA2R, ~70% of cases) or thrombospondin type 1 domain containing 7A (THSD7A, ~3% of cases). Antibodies directed against PLA2R or THSD7A can be monitored in the serum and correlate with disease activity (Sharma, 2018).
IgG, C3, and C5b-9 are detected in the electron dense immune deposits in membranous glomerulopathy. The presence of complement in deposits raises the possibility of complement activation as an effector mechanism and could implicate both the lectin pathway and alternative complement pathway. In the Heymann nephritis animal model of membranous glomerulopathy, antibodies to complement regulatory proteins play a role in the development of disease (Schiller, 1998). In a recent study, antibodies directed against complement factor H (CFH) were found to develop in a subset of patients with primary membranous glomerulopathy and can result in worsening renal function (Seikrit et al, 2018). In 93 screened patients, 3 were found to have a high titer of autoantibodies against CFH. Inhibition of CFH by autoantibodies at the surface of podocytes could result in increased activation of the alternative complement pathway and contribute to disease progression. Although not seen in a majority of patients, screening for CFH autoantibodies may be considered in patients refractory to therapy (Seikrit et al, 2018).
References:
Sharma SG, Larsen CP. Tissue staining for THSD7A in glomeruli correlates with serum antibodies in primary membranous nephropathy: a clinicopathological study. Modern Pathology 218; 31: 616-622.
Schiller B, He CL, Salant DJ, Lim A, Alexander JJ, Quigg RJ. Inhibition of complement regulation is key to the pathogenesis of active Heymann nephritis. The Journal of Experimental Medicine 1998 Oct 5; 188 (7): 1353-1358.
Seikrit C, Ronco P, Debiec H. Factor H autoantibodies and membranous nephropathy. New England Journal of Medicine 2018 December 20; 379: 2479-2481.
Quick note: This post is to be used for informational purposes only and does not constitute medical or health advice. Each person should consult their own doctor with respect to matters referenced. Arkana Laboratories assumes no liability for actions taken in reliance upon the information contained herein.

